
Improving medical management of Parkinson’s disease: Improving lives
Novel approaches to better manage Parkinson’s disease
In less than 10 years of treatment with the gold-standard antiparkinsonian medication, Levodopa/carbidopa, 9 in 10 individuals with Parkinson’s disease will develop dyskinesia
Dyskinesia is a near-inevitable side-effect of Levodopa treatment characterized by erratic, sometimes painful, involuntary movements that worsen with disease progression
As many as 9 in 10 people with Parkinson’s disease experience an erratic, sometimes painful involuntary movement side-effect known as dyskinesia within 10 years of treatment with the gold-standard antiparkinsonian medication, Levodopa/carbidopa.
Approach
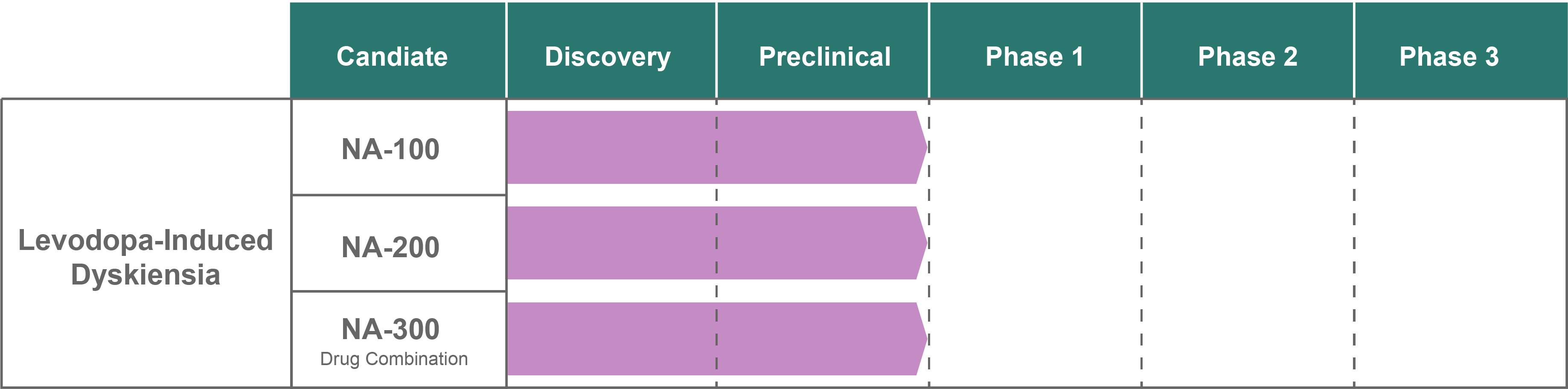
By using a unique combination of FDA-approved drugs that alleviate dyskinesia in well-validated preclinical models of Parkinson’s Disease, our therapeutics already have a validated affinity for our targets, a proven safety and tolerability profile in Parkinson’s patients, and a shorter pathway-to-market. All of this will enable Neuralina Therapeutics to provide patients with rapid access to effective anti-dyskinetic drugs.
Improving quality of life for
people with Parkinson’s disease.
Team

Per Stromhaug
Associate Vice President for Innovation and Economic Development
Per Stromhaug oversees the office of Entrepreneurship and Innovation Partnerships, including tech-transfer activities, the Start-up Suite, entrepreneurial support and economic growth initiatives. He also oversees the Koffman Southern Tier Incubator in downtown Binghamton and is the lead contact for the START-UP NY initiative at Binghamton University. (extracted from https://www.binghamton.edu/research/innovation/staff.html)

Olga Petrova
Assistant Director of Entrepreneurship and Innovation Partnerships
Olga serves as the Assistant Director for the Office of Entrepreneurship and Innovation Partnerships at Binghamton University. She has over 10 years of experience in molecular microbiology, including a Postdoctoral Fellowship through the Pharma Research and Early Development (pRED) division of F. Hoffmann-La Roche. In her current role, she manages the Binghamton University life sciences and biomedical technology portfolio and assists with the management of the Upstate Medical University technology portfolio, focusing on intellectual property protection, commercialization of new technologies, federal and industry grant proposal preparation, entrepreneurship, and startup company development. In addition, she directs the academic pre-accelerator XCEED program and serves as an instructor for the National Science Foundation Innovation Corps (NSF I-Corps) program.

Chris
Co-owner of patent
Chris is a full professor of Psychology and Director of the Integrative Neuroscience program at Binghamton University. He established his Movement Disorders Laboratory in 2005 after receiving his PhD and post-doctoral training at Wayne State University. His translational work using rodent models of disease has received support from both federal and foundation sources leading to the identification of several targets for the treatment of Parkinson’s disease. Recently, Chris has been overseeing the validation and translation of several drug entities that have promise as repurposed molecules for optimizing Parkinson’s disease treatment with L-DOPA, including the present technology invention. Serving as the PI, Chris will support the Targeted Neural Therapeutics team through his knowledge of Parkinson’s disease, his expertise in preclinical validation studies and overlapping support via extant and prospective funding.

Fredric
Principle Investigator of SBIR Grant
Fredric is an Associate Professor in Translational Neuroscience at Barrow Neurological Institute, where he runs a research group focused on the etiology and symptomology of neurodegenerative disease, primarily Parkinson’s disease (PD). His laboratory is currently funded by the NIH, DOD, and various foundations to develop novel therapeutic modalities for diseases such as PD. One such therapeutic was identified in collaborative and parallel work with Dr. Bishop. Fredric utilized genetic means to validate the targeting of specific serotonin receptors in improving the response of PD patients to medication, validating the pharmacological data collected by Dr. Bishop. Together, Drs. Bishop and Manfredsson identified several drugs for rapid repurposing and clinical testing, and are now actively looking for funding in the form of entrepreneurial investments.

Michael
Principle Investigator of SBIR Grant
Michael has graduated from Binghamton University in 2019 with his BSc in Integrative Neuroscience and Biochemistry and minors in Evolutionary Science and the BU Scholars Program. Previously he has worked as an undergraduate research assistant in Bishop Laboratory of Movement Disorders, choosing to stay year-round to further investigate dyskinesia in a hemiparkinsonian model. Thereafter Michael continued his research in the Bishop Lab and began investigating the commercial potential of several therapeutics that were previously studied in the lab. Leveraging his membership within the Society for Neuroscience and the Movement Disorders Society, Michael has begun to develop a network of useful connections through customer discovery with patients, general practitioners, Movement Disorders Specialist Neurologists, and industry executives. Throughout customer discovery, Michael continues to uncover the unmet medical needs of patients and their associated market demands.





